Abstract
Introduction
ADAMTS13 activity (ADAMTS13:Ac) measurement is an important diagnostic marker in acute TTP (defined as a level of <10%). Increasing numbers of commercial assays have been developed (ELISA, FRET and chemiluminesence) for diagnostic purposes. The International Council for Standardization in Haematology have not made specific recommendations on ADAMTS13 assay selection in a recent publication in 2020.
ADAMTS13:Ac has traditionally been used for diagnostic purposes but can also be useful for monitoring disease status and relapse risk. Patients between levels of 10-50% may require increased monitoring and subsequently treatment to avoid frank relapse, with an inverse relationship between ADAMTS13:Ac levels and intensity of clinical monitoring.
We seek to compare the differences between different commercial assays available to measure the ADAMTS13:Ac in patients who have a history of TTP and whether this influences clinical practice.
Methods
Patients with an acute diagnosis of TTP as an inpatient or outpatient surveillance with past history of TTP were reviewed at Guy's and St Thomas' Hospital, London, UK, and samples were taken to measure ADAMTS13:Ac as part of disease monitoring between February and July 2021. A comparison was performed between an ELISA (Technozym ADAMTS13 activity, Technoclone) and FRET (Technofluor, Technoclone), with all samples processed in parallel. With no international consensus, broadly, levels of <10% were defined as either active TTP or relapse; patients with 10-30% required more intensive outpatient monitoring to detect for relapse and >50% was defined as within the normal range.
Results
47 samples from 31 known or newly diagnosed TTP patients were processed over a six month period. Intra- and inter-assay CV for both ELISA and FRET on laboratory testing was as expected from manufacturers guidelines.
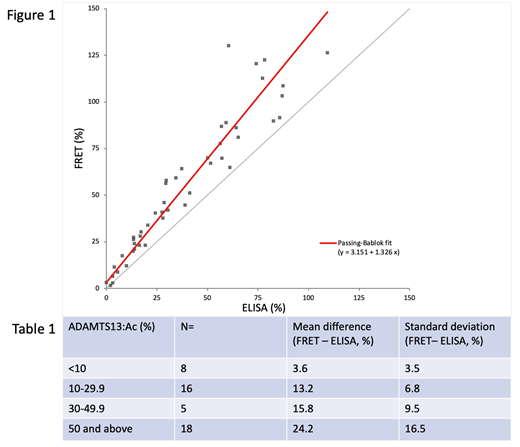
Figure 1 plots the ADAMTS13:Ac ELISA vs FRET, with a Pearson correlation of p=0.949 across all samples. Ranges were for ELISA (0 - 109.3) and FRET (1.6 - 130.3). Mean ADAMTS13:Ac difference was 16±13.5%, with consistent positive bias in favour of the FRET assay. Higher levels demonstrated greater variability as demonstrated in Table 1, with the mean difference between ELISA and FRET increasing with increasing levels.
Based on the above definitions as in the methods, there were 14/47 (29%) samples which had discordant results between the ELISA and FRET methods: 3 discordant samples on diagnosis of relapse, 8 discordant for where the ELISA detected levels <30% and FRET >30%; and 2 discordant for where ELISA <50% and FRET >50%.
Conclusion
Real-world clinical testing demonstrates substantial variability within a single manufacturer between two separate methods and demonstrates an impact on patient follow up and implications in decisions on follow-up intervals and treatment. There was a consistent positive bias in ADAMTS13:Ac by FRET measurement as compared with the ELISA. Greater consistency was demonstrated at the lower end of testing, where there was good concordance at levels of <10%, confirming that both assays were effective at determining a diagnosis or relapse of TTP. Further interrogation of other commercial platforms is warranted to establish the variability of results to inform on clinical practice in the monitoring of patients with TTP.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal